Background
Limited stage (stage I−II) disease is an uncommon presentation of mantle cell lymphoma (MCL), and its optimal management remains controversial. A British Columbia study (Leitch, Ann Oncol, 2003) and a Surveillance, Epidemiology and End Results (SEER) study (Murthy, Clin Lymphoma Myeloma Leuk, 2014) suggested the importance of radiotherapy (RT). A National Cancer Database (NCDB) study in the pre-BTK inhibitor (BTKi) era showed that chemotherapy (Chemo) plus RT (Chemo+RT) improved overall survival (OS) compared to Chemo or RT alone (Gill, Int J Radiat Oncol Biol Phys, 2015). However, a retrospective International Lymphoma Radiation Oncology Group study (Dabaja, Ann Oncol, 2017) reported comparable OS with Chemo+RT vs Chemo or RT alone. The potential differences in OS with different frontline therapy modalities may be modified by subsequent therapies for relapsed or refractory (R/R) disease. In this study, we used the NCDB data to analyze frontline treatment patterns and outcomes of limited stage MCL in the pre-BTKi vs BTKi eras.
Methods
The NCDB was queried to identify patients with stage I−II MCL diagnosed in 2004−2020. Because the first BTKi ibrutinib was approved by the FDA for R/R MCL in November 2013, we defined 2004−2013 as pre-BTKi era and 2014−2020 as BTKi era. Frontline treatment modalities were categorized to RT alone, Chemo alone, or Chemo+RT. Survival analysis was performed using the Kaplan-Meier method and the Cox proportional hazards model.
Results
Among 32,746 patients with newly diagnosed MCL in 2004−2020, 3,836 (11.7%) presented with stage I−II disease, with 2,428 having treatment information available. For patients diagnosed in the pre-BTKi era (n = 2005), 195 (9.7%) received RT alone, 1472 (73.4%) received Chemo alone, and 338 (16.9%) received Chemo+RT. For patients diagnosed in the BTKi era (n = 423), 45 (10.6%) received RT alone, 320 (75.7%) received Chemo alone, and 58 (13.7%) received Chemo+RT. Patients in the RT alone group were older (P <0.001) and had more females (P <0.001), but there were no statistically significant differences in Charlson-Deyo score, international prognostic index, or treating facility type (academic vs non-academic).
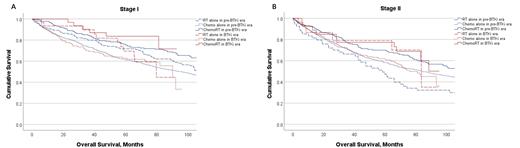
For patients with stage I disease, in the pre-BTKi era, patients treated with Chemo+RT (n = 182) had improved OS compared to those treated with RT alone (n = 141) or Chemo alone (n = 647), with a 5-year OS of 75.4% vs 71.8% vs 61.4% (P <0.001). Similar results were observed in the BTKi-era, with a 5-year OS of 83.6% vs 69.5% vs 62.9% (P <0.001) for patients treated with Chemo+RT (n = 31), RT alone (n = 30), or Chemo alone (n = 130), respectively. After adjusting for age and sex, Chemo+RT was associated with improved OS compared to Chemo alone (pre-BTKi era, HR = 0.59, 95% CI = 0.46−0.75, P <0.001; BTKi era, HR = 0.34, 95% CI = 0.14−0.79, P = 0.013), but not compared to RT alone (pre-BTKi era, HR = 0.62, 95% CI = 0.23−1.68, P = 0.343; BTKi era, HR = 0.77, 95% CI = 0.56−1.05, P = 0.10) (Figure 1A).
For patients with stage II disease, in the pre-BTKi era, patients treated with Chemo+RT (n = 156) had improved OS compared to those treated with RT alone (n = 54) or Chemo alone (n = 825), with a 5-year OS of 70.0% vs 47.3% vs 58.4% (P <0.001). In the BTKi-era, patients treated with Chemo+RT (n = 27) or RT alone (n = 15) both had improved OS compared to those treated with Chemo alone (n = 190), with a 5-year OS of 77.2% vs 78.8% vs 61.9% (P = 0.004) . After adjusting for age and sex, Chemo+RT was associated with improved OS compared to Chemo alone in BTKi era only (pre-BTKi era, HR = 0.85, 95% CI = 0.67−1.07, P = 0.165; BTKi era, HR = 0.49, 95% CI = 0.25−0.95, P = 0.034), but not compared to RT alone (pre-BTKi era, HR = 0.78, 95% CI = 0.53−1.13, P = 0.185; BTKi era, HR = 0.97, 95% CI = 0.35−2.67, P = 0.952) (Figure 1B).
Conclusions
Treatment pattern for limited stage MCL has not changed significantly from the pre-BTKi era to the BTKi era, with approximately 15% of patients receiving Chemo+RT, 10% RT alone and 75% Chemo alone. In the BTKi era, Chemo+RT appeared to be associated with better OS compared to Chemo alone but not compared to RT alone. Interpretation of the results requires caution because (1) patient numbers are small in Chemo+RT and RT alone groups, (2) longer follow-up may be needed, and (3) selection of therapy was not random, eg, patients receiving Chemo alone may have had higher disease burden such as bulky disease and/or non-contiguous stage II involvement but these details are not available in NCDB.
Disclosures
Paludo:Biofourmis: Research Funding; AbbVie: Consultancy; Karyopharm: Research Funding. Munoz:TG Therapeutics: Consultancy; Alexion: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Epizyme: Consultancy; Pfizer: Consultancy; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Incyte: Research Funding; Merck: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding; Curio: Honoraria; MEI: Consultancy; Portola: Research Funding; Lilly/Loxo: Consultancy; Beigene: Consultancy, Research Funding, Speakers Bureau; OncView: Honoraria; Targeted Oncology: Honoraria; Millennium: Research Funding; ADC Therapeutics: Consultancy; Kyowa: Honoraria, Speakers Bureau; Physicians' Education Resource: Honoraria; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Verastem: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Celgene: Research Funding. Ansell:ADC Therapeutics, Affimed, Bristol-Myers Squibb Company, Pfizer Inc, Regeneron Pharmaceuticals Inc, Seagen Inc, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research. Habermann:Genentech: Research Funding; sorrento: Research Funding; BMS: Research Funding. Witzig:Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Kura Oncology: Research Funding. Nowakowski:Kite Pharma: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Zai Lab Limited: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kymera Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Selvita Inc: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Incyte: Consultancy; Debiopharm: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Seagen: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; TG Therapeutics: Consultancy; Curis: Consultancy. Wang:Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; Novartis: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal